Focus Area
Ulcerative colitis (UC) is one of the two most frequent inflammatory bowel diseases. The onset age of UC peaks between 15 and 30 years and between 50 and 70 years. The condition is chronic and dominated by episodes of bloody diarrhea and intermittent periods of remission with quiescent disease. In the industrialized world, the prevalence of UC is approximately 250 per 100.000 persons. The incidence of UC is increasing in newly industrialized countries that adopt a western lifestyle. On a global scale, approximately 2.5 million people suffer from UC. In addition, UC is associated with an increased risk of colorectal cancer.
Severe acute flare-ups of UC are life threatening and are treated surgically by complete removal of the large intestine. Nevertheless, although curative, the surgical option is usually the last choice and obviously not a desirable solution. Less severe cases are treated medically using small molecule-based drugs with anti-inflammatory properties. Anti-inflammatory biological therapy is also used for a fraction of patients.
About us
EnteroTarget is a spinout company from the University of Copenhagen established in 2018 after the discovery of a novel mechanism involved in the regeneration of the colonic epithelial lining. It is the goal to partner up with potential investors to bring this therapy to the market with ulcerative colitis as the primary medical indication and Crohn’s disease as a potential additional indication. EnteroTarget holds an exclusive worldwide license to the invention.
Mission
Despite the availability of several anti-inflammatory drugs, a significant fraction of patients are refractory to treatment and maintains high disease activity. These patients are at increased risk of undergoing colectomy or other intestinal resections. The limitations of existing therapies, thus, necessitate other alternatives, mainly with regard to the epithelium. Reinforcing the mucosal barrier by targeting epithelial lining could potentially exclude many of the detrimental events caused by the accumulation of epithelial damage that leads to pathogenic invasions and mucosal ulcerations.
Our mission is to address this unmet medical need and develop a novel therapeutic strategy to treat inflammatory conditions of the intestinal tract, mainly the large intestine.
Technology
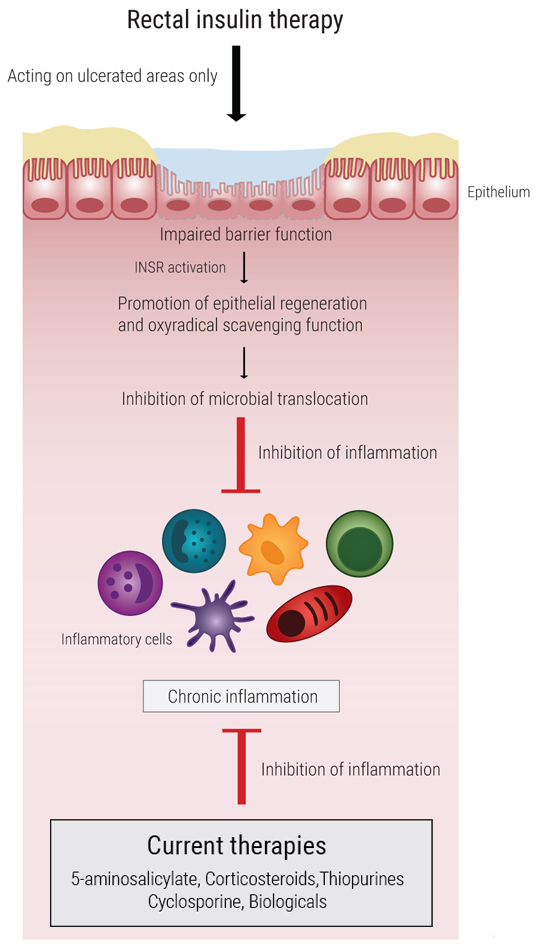
Although mucosal healing is the desired long-term outcome of IBD therapy, the majority of available treatment options controls disease symptoms by targeting the immune system rather than the epithelium. Yet, this strategy is still to be exploited in the clinic due to the lack of drugs that specifically target the intestinal epithelial wall without potentially initiating/promoting cancer. Our findings in vivo identify the intestinal epithelial insulin receptor as such a novel targeting candidate.
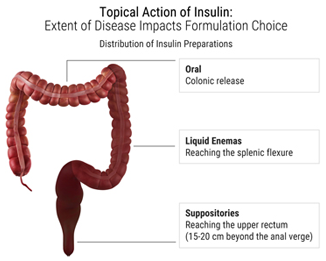
EnteroTarget’s technology relies on the administration of insulin to the lumen of the colon by either rectal administration or by oral administration in a delayed release form.
The main unique advantage is that rectal insulin therapy achieves an anti-inflammatory effect by targeting the epithelial cells rather than the immune cells and can thus synergize with existing IBD therapies. Another advantage is that topical insulin administration acts locally at the site of disease, and thus reduces the risk of any undesired systemic side effects. Moreover, the pharmacological properties of human insulin are already well-known and insulin can be self-administered which is more convenient for the patients, thus reducing costs related to hospitalization and improving quality of life and flexibility for the patients.
The team
The team behind EnteroTarget ApS
Prof. Jørgen Olsen
Co-founder
CEO
Professor, MD DMSc
Prof. Olsen is the co-inventor of the technology and the former director of the School of Medical Sciences and the medical study (UCPH). He studies the intestinal morphogenesis and differentiation. Since the millennium he has focused on genome wide gene expression studies and on metabonomics studies using colonic tissue from patients with IBD.
jo@enterotarget.com
Dr. Mohammad Yassin
Co-founder
Member of the Board
Medical Affairs Leader, PhD
Dr. Yassin is the co-inventor of the technology and has extensive experience from R&D and Medical Affairs. He is an expert in studies on intestinal inflammation and mucosal healing. Since 2018, Dr. Yassin has been working in top tier pharmaceutical companies with increasing Medical Affairs responsibilities in the field of IBD and Immunology and the current trends in the IBD treatment landscape and clinical unmet need.
my@enterotarget.com
Prof. Jesper Troelsen
Co-founder
Chairman of the Board
Professor of Medical Biology (RUC), PhD DMSc
Prof. Troelsen is a world-leading expert in the regulation of gene expression in the intestine and have since 1989 studied the biology of intestinal epithelial cells. Prof. Troelsen has discovered and characterized several genes and proteins involved in normal intestinal homeostasis, intestinal inflammation and colon cancer development.
jt@enterotarget.com
Prof. Anette Müllertz
Scientific Advisor - Oral Drug Delivery
Professor Physiological Pharmaceutics (UCPH), MSc PhD
Professor Müllertz has co-authored more than 200 papers within the field of drug delivery. Areas of expertise include innovative pharmaceutical formulations, dissolution and solubilization mechanisms for poorly soluble drug, media simulating the gastro intestinal fluids.
Dr. Aditi Singh
Advisor
Advisor - Marketing & commercialization
Senior Consultant
Dr. Aditi Singh has experience as:
Director, Business Development & Marketing, Infusion Care, Convatec Infusion Care
Global Project Lead, Diabetes Commercial Unit, Commercial Stragety & Affairs, Novo Nordic A/S
Project Lead, Merck
Sebastien Pratt
Member of the Board
Investment management
Over 17 years investment management experience and holder of the Investment Management Certificate from CFA UK. Sourcing and identifying attractive venture and private equity deals plus niche asset classes.
sp@enterotarget.com
Prof. Thomas Jonassen
Member of the Board
Co-founder and current CSO and BoD member at SynAct Pharma AB, cofounder of ResoTher Pharma Aps, cofounder and former CSO at Action Pharma A/S, and cofounder of TXP Pharma AG. Action Pharma sold its lead drug development candidate to AbbVie for $110M USD and TXP Pharma sold various rights to Questcor Pharmaceuticals for $100M USD in milestone payments. Dr. Jonassen is coinventor of SynAct’s drug candidate, AP1189.
Anna Polak-Andersen
Head of Clinical Development
Over 17-years of clinical trial oversight experience, clinical development programs within biotech, including six programs where the investigational medicinal products won the FDA/EMA regulatory approvals, extensive and active clinical trial design and conduct knowledge including early-stage projects, therapeutic expertise in autoimmune diseases, such as Ulcerative Colitis, Rheumatoid Arthritis, Atopic dermatitis well as rare diseases, Neurology and Oncology.
ap@enterotarget.com
News
EnteroTarget is pleased to announce the appointment of Anna Polak-Andersen as the new Head of Clinical Development. With an extensive background in clinical development at across biotechs as well as global organizations such as Medpace or Pfizer, Anna brings a wealth of expertise and a proven track record of success in auto-immune diseases including Inflammatory Bowel Disease (IBD).
During her 17-year career in the pharmaceutical industry R&D she has been actively involved in driving and developing clinical development programs including six programs where the investigational medicinal products won the FDA/EMA regulatory approvals.
In her new role, Anna will spearhead the development of EnteroTarget’s clinical trial strategy and ensure the optimal design and execution of future clinical studies. She will serve as the primary liaison to the European Medicines Agency (EMA), conducting early consultations to ensure the company’s clinical programs are aligned with regulatory requirements and maintain agility.
One of Anna's immediate priorities will be overseeing the planned Phase 1a safety study for patients suffering from Inflammatory Bowel Disease (IBD), in collaboration with the company’s selected Contract Research Organization (CRO). She will also lead the clinical work to advance a first-in-class IBD treatment for left-sided UC with ET enema & shuttle and Pouchitis with potential orphan designation.
Anna Polak-Andersen commented:
“I am thrilled to have been invited by Jørgen Olsen to join the ambitious EnteroTarget team. In a relatively short time, the company has made remarkable strides. It’s a team of scientists and entrepreneurs committed to excellence while maintaining a strong pace, and I am excited to be part of this journey.”
Jørgen Olsen, CEO of EnteroTarget, added:
“We are delighted to welcome Anna to our team. Her extensive experience in clinical operations makes her the ideal fit as we move into the next phase of development. There remains a significant unmet need for patients with Inflammatory Bowel Disease, many of whom do not find sufficient relief with current treatments. Together, we aim to bring new hope and innovative therapies to these patients.”
EnteroTarget Strengthens its Board with Professor Thomas Jonassen, MD
EnteroTarget is also proud to announce the appointment of Professor Thomas Jonassen, MD, an esteemed leader in biotech, to its board of directors. Thomas serves as an Associate Professor of Cardiovascular Pharmacology at the University of Copenhagen and has published over 50 scientific papers. He is also the inventor of six granted patents in the US and Europe.
With deep expertise in drug development and commercialization, Thomas is a co-founder of several biotech companies, including SynAct Pharma AB, ResoTher Pharma ApS, TXP Pharma AG, and former CSO of Action Pharma A/S. Action Pharma successfully sold its lead drug candidate to AbbVie for $110M, while TXP Pharma negotiated rights with Questcor Pharmaceuticals for up to $100M in milestone payments.
“We are fortunate to have Thomas join our board. His vast experience in drug commercialization and deep knowledge of the biotech landscape will be invaluable as we advance our clinical programs. Thomas’ entrepreneurial spirit, combined with his successful track record of transitioning early-stage assets to commercial pharma companies, is precisely the expertise we need as we continue to grow,” says Jørgen Olsen.
Entered into collaboration with the Polish insulin manufacturer, Bioton SA, as provider of insulin for EnteroTarget's Enterocalm products.
Initiated collaboration with Bioneer A/S for development of colonic release capsule.
InnoBooster Grant €120k for development of a capsule for oral administration.
Professor Jens Juul Holst, Professor Britta Siegmund and Dr. Johan Burisch recruited as Key Opinion Leaders for EnteroTarget and the development of rectal insulin therapy.
Read their opinion here.
Professor Anette Müllertz recruited as Scientific Advisor for the development of colonic drug delivery formulation.
CEO Jørgen Olsen will be pitching investment possibilities in EnteroTarget at Nordic Innovation Fair 21 September 2021.
EnteroTarget received InnoBooster Grant from Innovation Fund Denmark for "Insulin, Novel IBD Therapy" (DKK 484.400)
Our research mentioned in the media:
- University of Copenhagen: "Insulin viser stort potentiale mod kronisk tarmbetændelse"
- DR: "Maveproblemer: Insulin kan forhindre kroppen i at angribe tyktarmen"
- Dagens Pharma: "Insulin kan have stort potentiale til behandling af kronisk tarmbetændelse"
- Science Daily: "Insulin shows great potential against chronic colitis"
- Futurity: "Different kind of insulin injection may treat chronic colitis"
- RD MAG: "Insulin Shows Great Potential Against Chronic Colitis"
- Medical Xpress: "Insulin shows great potential against chronic colitis"
- Laboratory Equipment: "Insulin Shows Great Potential Against Chronic Colitis"
- Deccan Chronicle: "How insulin could help treat chronic colitis"
Our paper "Rectal Insulin Instillation Inhibits Inflammation and Tumor Development in Chemically Induced Colitis" was published in Journal of Crohn's and Colitis.